Here in all the compounds we see covalent bonds, that is to say that the atoms are sharing electrons with each other. Now, covalent bonds are divided into single, double or triple according to the number of electrons they share.
When they share a pair of electrons, a line is drawn that represents a bond. If it has only one it will be a simple bond, if it has two it will be a double bond and if it has 3 it will be a triple bond.
Here we see simple covalent bonds and double covalent bonds.
Now, to calculate the enthalpy change we can do it starting from the bond energies, applying the following formula:
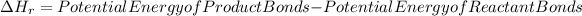
The bonds to take into account for the calculation of enthalpy will be:
Ethanol:
5 bonds C-H
1 bond C-O
1 bond O-H
Oxygen:
1 bond O=O, but there are 3 moles of O2, so it will be 3 bonds O=O
Carbon dioxide:
2 bonds O=C, there are 2 moles of CO2, so it will be 2x2= 4 bonds O=C
Water:
2 bonds O-H, there are 3 moles of H2O, so it will be 2x3=6 bonds O-H