Answer: The volume of the gas at STP is 45.0 L. hence the correct answer is option(2).
Step-by-step explanation:
Initial volume of the gas,
 = 50.0 L
= 50.0 L
Initial temperature of the gas
 =
=
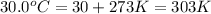
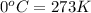
Final volume of the gas,
 = ?
= ?
At STP, the value of temperature is 273 K.
Final temperature of the gas
 = 273 H
= 273 H
Charles' Law: The volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
 (At constant pressure and number of moles)
(At constant pressure and number of moles)
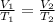
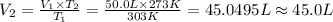
The volume of the gas at STP is 45.0 L. hence the correct answer is option(2).