molar mass CH4 = 12 + 1 . 4 = 16 g/mol
1 mole CH4 ------------ 16 g
5 moles CH4 ---------- x g
x = 5 . 16
x = 80 g
16 g CH4 ------------------ 6,02.10²³ atoms
80 g CH4 ------------------ y atoms
16 . y = 80 . 6.02x10²³
16 y =
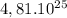
y =
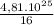
y =
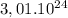
atoms
hope this helps