Answer:
67.58%
Step-by-step explanation:
First we convert the masses of the reactants into moles, using their respective molar masses:
- Na ⇒ 13.1 g ÷ 23 g/mol = 0.570 mol
- Cl₂ ⇒ 94 g ÷ 70.9 g/mol = 1.32 mol
By looking at the reaction equation, we know that 1.32 moles of Cl₂ would react completely with (2 * 1.32) 2.64 moles of Na. There are not as many Na moles, so Na is the limiting reactant.
Now we calculate how many NaCl moles would have been produced if all the limiting reactant was consumed:
- 0.570 mol Na *
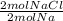 = 0.570 mol NaCl
= 0.570 mol NaCl
We convert those moles to grams:
- 0.570 mol * 58.44 g/mol = 33.31 g NaCl
Finally we calculate the percent yield:
- 22.5 g / 33.3 g * 100% = 67.58%