Answer: The radiation that is emitted from the above process is alpha particle.
Step-by-step explanation:
Alpha decay is defined as the process in which alpha particle is emitted. In this process, a heavier nuclei decays into a lighter nuclei. The alpha particle released carries a charge of +2 units. The released alpha particle is also known as helium nucleus.

Beta decay is defined as the process in which beta particle is emitted. In this process, a neutron gets converted to a proton and an electron. The released beta particle is also known as electron.

Gamma decay is defined as the process in which an unstable nuclei gives off excess energy by a spontaneous electromagnetic process and thus releases
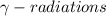 .
.
These radiations does not carry any charge and are electrically neutral.

The chemical equation for the decay of polonium-212 isotope follows:
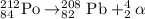
Hence, the radiation that is emitted from the above process is alpha particle.