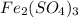Answer: There are 3 moles of sulfur atoms present in 1 mole of
Step-by-step explanation:
In the given compound
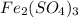 , there are 3 elements present: iron, sulfur and oxygen.
, there are 3 elements present: iron, sulfur and oxygen.
There are 2 moles of iron element, 3 moles of sulfur element and 12 moles of oxygen element present in 1 mole of
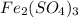
Hence, there are 3 moles of sulfur atoms present in 1 mole of