Answer:

Explanation:
A precipitation reaction is a special type of double displacement reaction.
 is an insoluble compound as it is does not dissolve in water, and thus formed as a soild or a precipitate.
is an insoluble compound as it is does not dissolve in water, and thus formed as a soild or a precipitate.
The sulfate ion
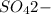 form insoluble compounds when combined with
form insoluble compounds when combined with
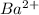 .
.
The chlorate ion
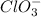 form soluble compounds with all positive ions.
form soluble compounds with all positive ions.
The chromate ion
 form insoluble compounds with all ions but soluble with
form insoluble compounds with all ions but soluble with
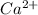 .
.
The sulfide ion
 form insoluble compounds with all ions except when combined with
form insoluble compounds with all ions except when combined with
 or
or
