Answer : The correct option is, (3) 400 K and 0.25 atm
Explanation :
The conditions for ideal gas are :
Ideal gas are those gas that has no intermolecular attractions.
Ideal gas are those gas that have negligible volume.
The ideal gas equation is,
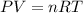
The conditions for real gas are :
Real gas are those gas that have intermolecular attractions.
Real gas are those gas that have volume.
The real gas equation is,

A real gas behave ideally at high temperature and low pressure condition.
From the given options, option (3) have high temperature and low pressure is the correct option.
Hence, the 400 K and 0.25 atm conditions of temperature and pressure does a sample of neon behave most like an ideal gas.