Answer: Option (3) is the correct answer.
Step-by-step explanation:
Average kinetic energy is defined as the mean of all the energies present within the molecules of a substance.
Also, we known that relation between kinetic energy and temperature is as follows.
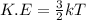
Since, kinetic energy is directly proportional to temperature. So, when there will be increase in temperature then this will also lead to increase in kinetic energy. Hence, average kinetic energy will also increase.
Thus, we can conclude that a change from
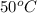 to
to
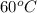 in the temperature of a 1-gram sample of water would cause the greatest increase in the average kinetic energy of its molecules.
in the temperature of a 1-gram sample of water would cause the greatest increase in the average kinetic energy of its molecules.