HCl reacts with NaHCO₃ this way:
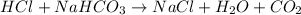
The equation is already balanced, it means that 1 mole of HCl reacts with 1 mole of NaHCO₃.
Convert the mass of NaHCO₃ to moles using the molecular mass of NaHCO₃ which is 84g/mol.
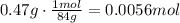
Now, use the ration stated before to find how many moles of HCl react with this amount of NaHCO₃:
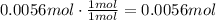
It means that 0.0056 moles of HCl are needed to react with 0.47g of NaHCO₃.