Answer:
a. "one mole of any substance has 6.022x10²³ atoms, molecules or ions (Avogadro's number) into it"
b.
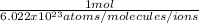 and
and
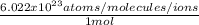
c.
•
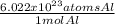
•
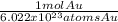
Step-by-step explanation:
Hello,
a. In this case, a plausible statement would be: "one mole of any substance has 6.022x10²³ atoms, molecules or ions (Avogadro's number) into it".
b. Now, the possible conversion factors could be:
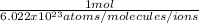
or conversely:
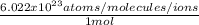
c.
• Moles Al from atoms Al: the conversion factor will be:
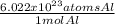
Since we will need to simplify moles to get atoms.
• Atoms Au from mol Au: the conversion factor will be:
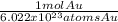
Since we will need to simplify atoms to get moles.
Best regards.