Answer: Option (d) is the correct answer.
Step-by-step explanation:
Atomic number of argon is 18 and its electronic configuration is
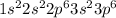 .
.
Also, it is known that number of electrons in K, L and M shells of argon are 2, 8, 8.
Thus, we can conclude that an atom of argon in the ground state tends not to bond with an atom of a different element because the argon atom has a total of eight valence electrons.
So, as per the octet rule, last sub-shell of argon is completely filled. Hence, it will not form any bond with any other atom.