Answer
The pressure, P inside the container (in atm) = 2.04 atm
Step-by-step explanation
Given:
Moles of the gas, n = 3.99 mol
Volume, V = 65.9 L
Temperature, T = 410 K
What to find:
The pressure, P inside the container (in atm).
Step-by-step solution:
The pressure, P inside the container (in atm) can be calculated using the ideal gas equation.

R is the molar gas constant and R = 0.0821 atm-liter / mol.K
Putting the values of the parameters into the formula, we have
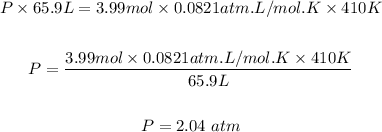
The pressure, P inside the container (in atm) = 2.04 atm