Answer: Option (1) is the correct answer.
Step-by-step explanation:
In a
 molecule, the nitrogen atoms are bonded together by triple bond.
molecule, the nitrogen atoms are bonded together by triple bond.
Therefore, in order to break this triple bond high heat or energy has to be provided.
Thus, the reaction
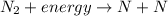 shows that N triple bond N is broken down as energy is absorbed by
shows that N triple bond N is broken down as energy is absorbed by
 molecule.
molecule.
Hence, we can conclude that in the given reaction bonds are broken, and the reaction is endothermic.