Answer: Glycerol has more attractive forces as compared to water.
Step-by-step explanation:
Boiling point is the temperature at which vapor pressure of the liquid becomes equal to atmospheric pressure.
Boiling point depends on the strength of inter molecular forces.
The molecules of glycerol
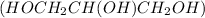 are more strongly bonded through hydrogen bonds as there are three OH groups.
are more strongly bonded through hydrogen bonds as there are three OH groups.
But in water
 , only on e OH group is present and thus extent of hydrogen bonding is less.
, only on e OH group is present and thus extent of hydrogen bonding is less.
Thus we can conclude that glycerol has more attractive forces as compared to water.