Hello!
An antacid tablet containing 0.50 g of NaHCO3 is dissolved in 250 mL of water. What is the molar concentration of NaHCO3 in the solution ?
- We have the following data:
M (Molar Concentration or Molarity) = ? (in mol/L)
m (mass) = 0.50 g
V (volume) = 250 mL → 0.25 L
MM (Molar Mass of NaHCO3)
Na = 1*(23u) = 23 u
H = 1*(1u) = 1 u
C = 1*(12u) = 12 u
O = 3*(16u) = 48 u
-------------------------------
MM (Molar Mass of NaHCO3) = 23 + 1 + 12 + 48 = 84 g/mol
- We apply the data found to the formula of molar Concentration or Molarity, let's see:
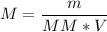
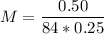
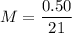
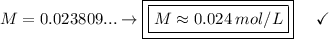
Answer:
The Molar Concentration is approximately 0.024 mol/L
________________________
