We have a gas that we will assume behaves like an ideal gas. So we can apply the ideal gas law. The ideal gas law tells us:

Where,
P is the pressure of the gas
V is the volume of the gas
n is the moles of the gas
R is a constant
T is the temperature of the gas
We have two states of the gas. One initial and one final, for both states it is assumed that the moles remain constant. The conditions for each state are.
Initial state:
V1=2.3mL
P1=5.3atm
T1=45°C =318.15K
Final state:
V2=1.2L
P2=2.5atm
T2=?
For each state the ideal gas law will be:
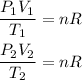
Now, as the moles remain constant, the term nR will be constant and we can equate the two equations, we will then have that:
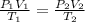
Now, we clear T2 and replace the known data:
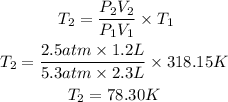
The temperature of the gas will be 78.3K