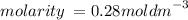Answer:
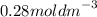
Step-by-step explanation:
given; 5.00g equivalent to 100mL
5.00g = 100mL
convert 100mL to dm³
100mL is 100milliLitre
milli = 10^-3
100mL = 100 × (10^-3)
= 10^2 × 10^-3
=
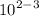
= 10^-1 L
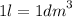
therefore, 10^-1 L = 10^-1 dm³
Relative Atomic Mass of;
C=12, H=1, O=16
Molar mass of glucose (C6H12O6)
= 12×6 + 1×12 + 16×6
= 180g/mol
Now convert the mass given to mole using;
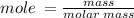
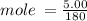
mole = 0.028 mol
therefore, 0.028 mol is equivalent to 10^-1 dm³
10^-1 dm³ = 0.028mol
divide both sides by 10^-1 to get 1dm³
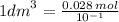
= 0.28 mol