Answer:
See explanation.
Step-by-step explanation:
Hello!
In this case, when writing the complete molecular, ionic and net ionic equations, we must make sure we have the reactants and the most likely products; thus, when lead (II) nitrate react with sodium sulfate, it is noticed that the complete molecular equation is:
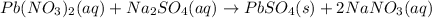
Whereas the one and only unionizable species is the lead (II) sulfate product; thus, the complete ionic equation turns out:
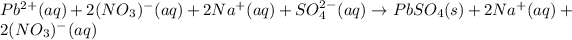
Whereas it is noticed that both sodium and nitrate ions are the spectator ions as they are present at both reactants and products; therefore, the net ionic equation turns out:
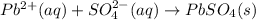
Best regards!