Answer:

Step-by-step explanation:
The formula for density is:
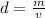
where m is the mass and v is the volume.
The mass is 0.45 kilograms and the density is 13.6 grams per milliliter. The density is given in grams, so we must convert the mass.
There are 1000 grams in 1 kilogram or
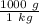 . We can multiply the mass by this ratio.
. We can multiply the mass by this ratio.
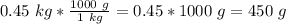
Now we have values for the mass and density:
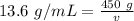
Cross multiply.
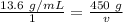
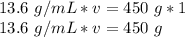
We are trying to find the volume, so we must isolate that variable.
13.6 and v are being multiplied. The inverse of multiplication is division. Divide both sides by 13.6
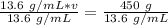
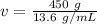
The grams will cancel.
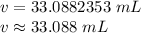
The volume is about 33.088 milliliters.