Hello!
What is the mass of 2.50 ×1022 molecules of NaOH (Molar mass = 40.0 g/mol)?
Data:
Molar Mass of NaOH = 40 g/mol
Solving: According to the Law Avogradro, we have in 1 mole of a substance, 6.02x10²³ atoms/mol or molecules
1 mol -------------------- 6.02*10²³ molecules
y mol -------------------- 2.50*10²² molecules
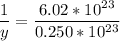
Product of extremes equals product of means
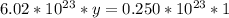
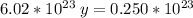
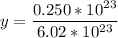
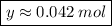
Solving: Find the mass value now
40 g ----------------- 1 mol of NaOH
x g ------------- 0.042 mol of NaOH
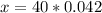

Answer:
The mass is 1.68 grams
_______________________
I Hope this helps, greetings ... Dexteright02! =)