Step-by-step explanation:
Number of moles consisting in a liter of solution is known as molarity.
Mathematically, Molarity = \frac{\text{no. of moles}}{\text{Volume in one liter}}[/tex]
It is given that volume is 10 liter and there is 5.0 moles of solute. Hence, we will calculate the molarity as follows.
Molarity =
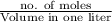
=
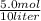
= 0.5 mol/L
Thus, we can conclude that molarity of the given solution is 0.5 mol/L.