Answer:
38.55L
Explanations:
Given the following parameters
Mass of propane = 25.3 grams
Determine the moles of propane
Moles of propane = Mass/molar mass
Moles of propane = 25.3/44.1
Moles of propane = 0.574moles
The combustion of propane is given according to the equation below:
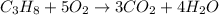
According to stoichiometry, 1mole of propane produces 3 moles of CO2, the moles of CO2 required will be:
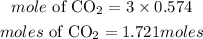
Since 1 mole = 22.4L, volume of CO2 gas that will be produced is:
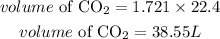
Hence the volume of carbon dioxide produced is 38.55L