There are 30 protons and 39 neutrons in the nucleus.
This must me the isotope of an element with an atomic mass close to 69 u.
The only candidates are Zn and Ga.
Zn has a zinc-69 isotope with mass 68.926 u.
Ga has a gallium -69 isotope with mass 68.925 u.
The isotope is probably
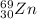
.
It has 30 protons and 39 neutrons.