For this, let's assume that classical expression for kinetic energy will work.
4.886 MeV = 7.8282347 x 10^-13 joules
7.8282347x10^-13 = 1/2(9.1093837 × 10-31)(v^2)
v = 1.311 x 10^9 m/s
The speed of light is 3 x 10^8
This means the electron should have been traveling faster than the speed of light, which is not possible. Classical Expression can't compute speed accurately.
For this we will need to use the relativistic equation
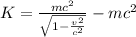
Where K is kinetic energy
Plugging in the numbers
7.828 x 10^-13 = (9.109 × 10-31)(3x10^8)^2/sqrt(1-(v^2)/(3x10^8^2)) - (9.109 × 10-31)(3x10^8)^2
Add the sides
(9.109 × 10-31)(3x10^8)^2/sqrt(1-(v^2)/(3x10^8^2)) = 8.65 x 10 ^-13
multiply by recripercal
(9.109 × 10-31)(3x10^8)^2/8.65 x 10 ^-13 = sqrt(1-(v^2)/(3x10^8^2))
.09477 = sqrt(1-(v^2)/(3x10^8^2))
Square both sides
0.00898 = 1-(v^2)/(3x10^8^2)
subtract the 1
(v^2)/(3x10^8^2) = 1-0.00898
(v^2)/(3x10^8^2) = .99101
multiply by denominator
v^2 = .99101*(3x10^8^2)
v^2 = 8.919x10^16
square root
v = 2.98 x 10^8 m/s
Convert to fraction of speed of light
v = 0.996c