Answer:
» Similarities :
☑ They both react by chemical means, to produce either an element or compound as the end product of the chemical reaction.
• Take an example of Magnesium reacting with cold water:
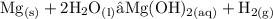
• If it reacts with steam:
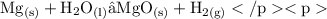
• [ Element: Magnesium and Compound: Water ]
They are both classified under atomic substances cause they are made up of microscopic molecules known as atoms.
☑ They are both formed up by microscopic substances known as atoms.
» Differences :
☑ Compounds generally have higher molecular masses than elements.
☑ Compounds are formed through bonding by elements while elements can naturally exist or formed by extraction, purification and concentration