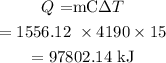Given that the temperature change of steel engine is
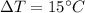
Let the mass of steel engine be denoted by m.
The weight of the steel engine is
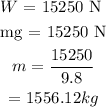
Also, the specific heat of coolant (water) is C = 4190 J kg/degC
We have to find the heat reduction.
The heat reduction can be calculated as