To determine the material we will determine the density using the values of mass and volume. We will use the following formula:
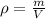
Where:
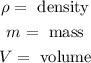
Now, we plug in the values:
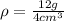
Solving the operations:
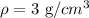
The closest material is the one with a density of:
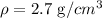
Therefore, the material could be aluminum.