Answer
b. 4.2 x 10²⁴
Step-by-step explanation
Given:
Moles of H2O = 7 moles
Avogadro's Number is 6 x 10²³
What to find:
The number of articeles of H2O present in 7 moles of H2O.
Solution:
1 mole f any substanc contains 6 x 10²³ partcicles
Therefore 7 moles of H2O will contain
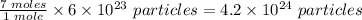
The correct answer is option:
b. 4.2 x 10²⁴