Answer: Option (D) is the correct answer.
Step-by-step explanation:
Molarity is number of moles divided by volume of the solution.
Mathematically, Molarity =
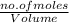
Also, in the given situation number of moles of both HCl and NaOH are equal. Therefore, volume of HCl will be calculated as follows.
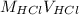 =
=
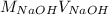
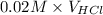 =
=

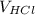 =
=
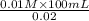
= 50 mL
Thus, we can conclude that volume of HCl is 50 mL.