Answer: The correct option is 3.
Explanation: Radioisotopes which emits alpha-particle are known as alpha-emitters. These radioisotopes undergo alpha-decay.
The radioisotopes which emits beta-particle
 are known as beta-emitters. These radioisotopes undergo beta-minus decay. In this decay a neutron gets converted to a proton and an electron.
are known as beta-emitters. These radioisotopes undergo beta-minus decay. In this decay a neutron gets converted to a proton and an electron.
The radioisotopes which emits positron-particle
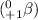 are known as positron-emitters. These radioisotopes undergo beta-plus decay. In this type of decay a proton gets converted to a neutron.
are known as positron-emitters. These radioisotopes undergo beta-plus decay. In this type of decay a proton gets converted to a neutron.
From the given options,
Option 1: All the three radioisotopes undergoes beta-minus decay.
Option 2: Cs-137 and Tc-99 radioisotopes undergo beta-minus decay.
Fr-220 is a radioisotope which undergoes alpha-decay.
Option 3: Radioisotope Kr-85 undergoes beta-minus decay.
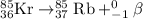
Radioisotope Ne-19 undergoes positron decay.
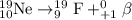
Radioisotope Rn-222 undergoes alpha decay.
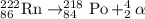
Option 4: All the three radioisotopes undergoes beta-minus decay processes.
Hence, from the above information, the correct option is 3.