Answer : Option 1) nuclei of
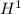 and nuclei of
and nuclei of
 only.
only.
Explanation : Radiation is spontaneously emitted from nuclei of
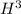 because this isotope of hydrogen is highly radioactive as compared to other isotopes of hydrogen namely; nuclei of
because this isotope of hydrogen is highly radioactive as compared to other isotopes of hydrogen namely; nuclei of
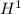 and nuclei of
and nuclei of
 .
.
They have much stable nucleus as compared to nuclei of
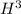 .
.
The more it is unstable the more radiations will be emitted from its nucleus.