You can solve this by using Charle's gas law:
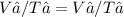
V = Volume and T = Temperature
Next you need to convert C to Kelvin, by adding 273 to the C temp.
10 + 273 = 283 and 20 + 273 = 293
Now all you do is plug in your numbers to the equation:
4.00L ÷ 283K = V₂ ÷ 293K
Next solve for V₂:
V₂ = (4.00 · 293) ÷ 283
V₂ ≈ 4.14L (You round to 3 significant figures because that is how many your initial volume had)