Answer:
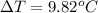
Step-by-step explanation:
Hello,
In this case, we consider such heat absorption as an energy inlet, that is why the temperature of the water will raise as shown below as a positive result is obtained, considering water's heat capacity as 4.18 J/(g*°C):
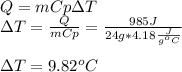
Best regards.