Answer: The nucleus of radium-226 is unstable and hence undergoes decay.
Explanation: Radium has many isotopes. One of them is
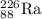 which has 88 protons and 138 neutrons. It is a radioactive isotope and undergoes decay process.
which has 88 protons and 138 neutrons. It is a radioactive isotope and undergoes decay process.
This isotope undergoes alpha - decay and produces Radon-222 isotope.
Equation for alpha - decay follows:
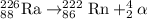
Hence, the nucleus of radium-226 undergoes decay process.