Answer: Option (2) is the correct answer.
Step-by-step explanation:
Electrolytes are the species that contain electric charges and are present as ions in a solution.
We can also say that ionic substances act as an electrolyte in a solution because ionic compounds have the ability to dissociate into ions.
For example,

and
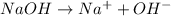 dissociate completly into ions when dissolved in wateror polar solvent.
dissociate completly into ions when dissolved in wateror polar solvent.
Thus, we can conclude that out of the given options, HCl and NaOH is the pair of formulas represents two compounds that are electrolytes.