Answer: (2) structural formula
Explanation:-
The compounds having similar molecular formula but different arrangement of atoms or groups in space are called isomers and the phenomenon is called as isomerism.
1-bromopropane and 2-bromopropane are position isomers which have same molecular formula but different position of the functional groups attached.
The chemical formula for both the compounds 1-bromopropane and 2-bromopropane is
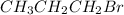 . Thus both have same molecular formula , 3 carbon atoms per molecule and 1 bromine atom per molecule.
. Thus both have same molecular formula , 3 carbon atoms per molecule and 1 bromine atom per molecule.