First step, is to find the Molar Mass.
If you go to the periodic table, the Molar Mass of the Sodium Na is 23g/mol
Second Step, you convert from grams to moles.
number of Mole = mass of sodium / molar mass =
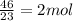
Third Step, you find the number of atoms.
As We know, Avogadro said that the number of atoms in 1 mole is about
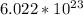
.
You use the Rule of three and you find the number of atoms!
1 mole =
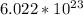
atoms
2 mole = x atomes
x = 2 * (
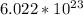
)
x=
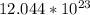
atomes
So, there is
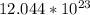
atoms of Sodium Na in 46g of Solution.
Hope this Helps! :D