Answer:
0.1784 moles of helium gas are contained in a 4.0-L flask at STP.
Step-by-step explanation:
At STP. temperature of the helium gas = T = 273.15 K
At STP, pressure of the helium gas = P = 1 atm
Volume of the helium gas at STP = V = 4.0 L
Moles of the helium gas = n
Using an ideal gas equation :
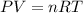

n = 0.1784 mol
0.1784 moles of helium gas are contained in a 4.0-L flask at STP.