1) List the known and unknown quantities.
Initial conditions
Volume: 2.5 L.
Pressure: 1.0 atm.
Temperature: 325.4 K.
Final conditions
Temperature: 275.9 K.
Pressure: 293.4 kPa.
Volume: unknown (mL).
2) Set the equation
The combined gas law.
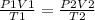
2.1- Convert kPa to atm.
101.325 kPa = 1 atm
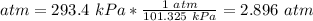
3) Plug in the known quantities
.
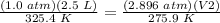
Multiply on both sides by 275.9 K.
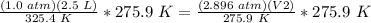
Divide both sides by 2.896 atm.
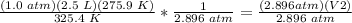
.
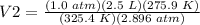
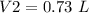
4) Convert L to mL.
1 L = 1000 mL
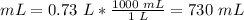
The final volume at the unknown depth is 730 mL.
.