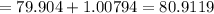Answer:
Molar mass of bromine is equal to
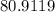
Step-by-step explanation:
The molar mass of HBr is equal to the sum of atomic weight of Bromine.
Atomic Weight of hydrogen is equal to
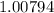
Atomic Weight of Bromine is equal to
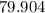
Molar mass of Bromine
= Atomic Weight of hydrogen + Atomic Weight of Bromine
Molar mass of Bromine