Answer:
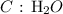
Step-by-step explanation:
Here, we want to get the limiting reactant
The limiting reactant is the reactant that produces less number of moles of the product
Firstly, we need to get the number of moles of each of the reactants
To get this, we divide the mass of the reactants by the molar mass of the reactants
The molar mass of Al2S3 is 150 g/mol
The number of moles is thus:
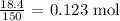
Now, let us get the number of moles in the products
For Aluminum hydroxide we have 2 * 0.123 = 0.246 mol
For Hydrogen Sulfide, we have 3 * 0.123 = 0.369 mol
Now, let us get for water
We start by getting the number of moles. We divide the mass of water given by the molar mass of water which is 18 g/mol
That gives us:
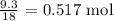
Now, from the equation of reaction:
6 moles of water gave 2 moles of Aluminum hydroxide
0.517 mol of water will give:
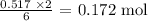
For Hydrogen sulfide:
We have the number of moles as:
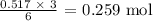
Now, comparing the number of moles, the number of moles of products produced by water is smaller and that means water is the limiting reactant