Answer:
Gas particle motion will drop as the temperature reaches 252 K
Step-by-step explanation:
Initial temperature T1 = 295 K
Final temperature, T2 = 252 K
The kinetic energy of the gas particles is related to the temperature via the following equation:
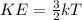
where k = Boltzmann constant
Based on the above equation we can write:
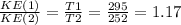
KE(1) = 1.17KE(2)
This implies that the kinetic energy is lower at T2 and therefore the gas particles will slow down as the balloon rises.