1) Balance the chemical equation.
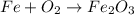
Balance Fe.
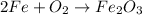
Balance O.
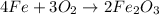
2) Moles of Fe2O3 produced from 1.15 mol Fe.
The molar ratio between Fe2O3 and Fe is 2 mol Fe2O3: 4 mol Fe.
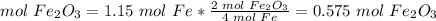
1.15 has three significant figures. So, this result, 0.575 has three significant figures.
3) Convert moles of Fe2O3 to grams of Fe2O3
The molar mass of Fe2O3 is 159.6882 g/mol
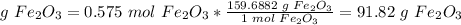
0.575 has three significant figures. So, this result, 91.82 must be rounded to three significant figures.
91.8 g Fe2O3 are produced from 1.15 mol Fe.
.