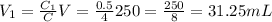You are right.Here's an easier way to do it :
We have an initial solution of concentration

.
We want to turn it into a

mL solution of concentration

, and we want to know which volume

of solution to take from the original to do that.
We know that
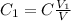
hence