Step-by-step explanation:
Osmotic pressure is defined as the minimum pressure which has to applied on the solution to prevent the entry of the solvent in the solution through a semi-permeable membrane.
It is a type of colligative property which means it depends on the amount of the solution. More the amount, more will be the osmotic pressure.
Mathematically,

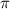 = Osmotic pressure
= Osmotic pressure
i = Vant's Hoff factor which is 1 for non-electrolyte solutions
c = Concentration of solution
R = Solution constant
T = temperature
- For

As Ferric chloride is an electrolyte, so it will completely dissociate into its ions.
1 mole of ferric chloride will dissociate into 1
 ion and 3
ion and 3
 ions.
ions.

Here, value of i = 4
Glucose is a non-electrolyte and hence will not dissociate into the respective ions. So, value of i = 1
Osmotic pressure will be high for high value of 'i'. Therefore, osmotic pressure of
 will be high as compared to the glucose in same amount of water.
will be high as compared to the glucose in same amount of water.