Answer:
2.85moles of oxygen gas
Step-by-step explanation:
Given parameters:
Volume of oxygen gas = 63.8L
Unknown:
Number of moles = ?
Solution:
We assume that the gas is under standard temperature and pressure. To find the number of moles, use the expression below:
1 mole of a gas at STP occupies a volume of 22.4L
So;
63.8L of oxygen gas will take up a volume of
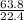 = 2.85moles of oxygen gas
= 2.85moles of oxygen gas