Answer:
4.1 moles of FeCl₃
Step-by-step explanation:
The reaction expression is given as shown below:
2Fe + 3Cl₂ → 2FeCl₃
Number of moles of Cl₂ = 6.1moles
So;
We know that from the balanced reaction expression:
3 moles of Cl₂ will produce 2 moles of FeCl₃
Therefore 6.1moles of Cl₂ will produce
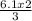 = 4.1 moles of FeCl₃
= 4.1 moles of FeCl₃
The number of moles is 4.1 moles of FeCl₃