Given data:
Mass number of the atom:
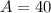
Number of neutrons;
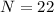
The mass of the atom is given as,
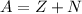
Here, Z is the number of protons in the atom.
Therefore, the number of protons in the atom is given as,
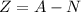
Substituting all known values,
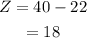
Therefore, there are 18 protons in the nucleus of the atom.
The number of electrons in a neutral atom is equals to the number of protons inside the nucleus.
Therefore, the number of electrons in the atom is 18.
The electronic configuration of the atom is given as,
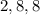
Therefore, the last shell have 8 electrons.
As, the last shell is completely filled, which means it has 8 electrons. So, the atom does not need any extra electron to comply with the octet rule.