Answer:
850g/min
Step-by-step explanation:
The reaction expression is given as:
P₄ + 5O₂ → P₄O₁₀
Given that:
Number of moles of product = 1.5moles
Time = 30s = 0.5min
Unknown:
The rate of the reaction = ?
Solution:
Convert the moles given to mass of the product.
So;
Mass of product = number of moles x molar mass
Molar mass of P₄O₁₀ = 4(31) + 10(16) = 284g/mol
Mass of product = 1.5moles x 284g/mol = 426g
So;
Reaction rate =
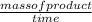 =
=
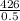 = 850g/min
= 850g/min